
BMC devotes in respiratory healthcare over 20 years, partner with families worldwide to overcome the discomfort of chronic respiratory disease with quality products, professional services, and proactive care, aimed to become the first choice in respiratory care.
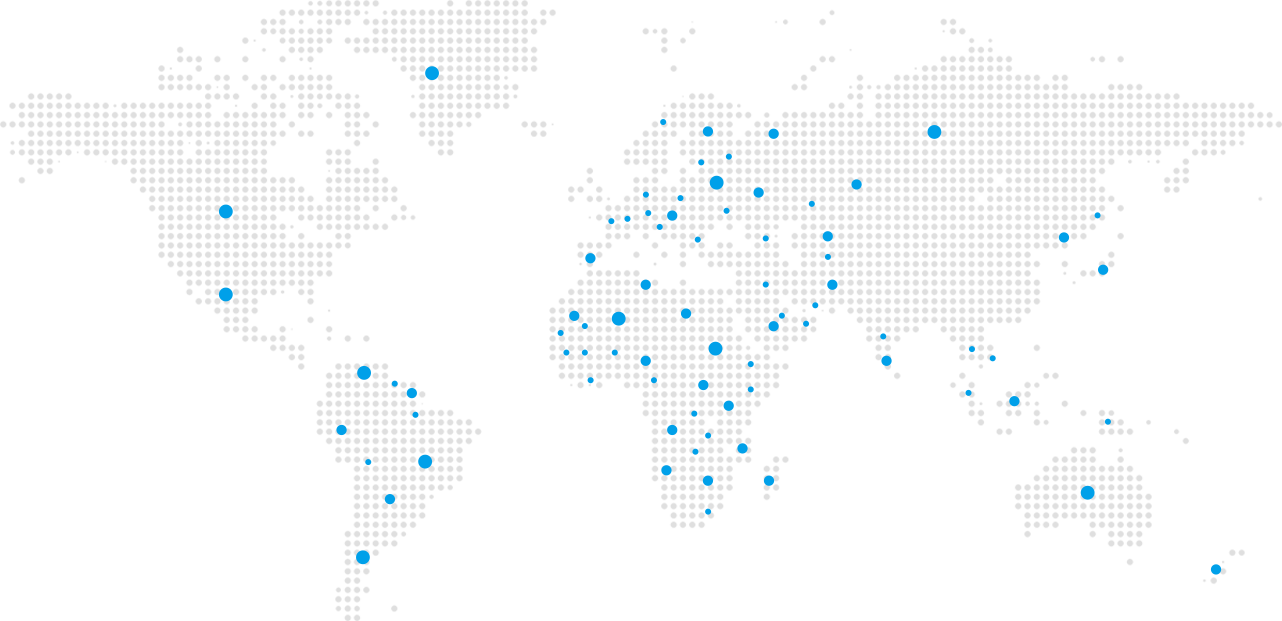









Over 20 products have been certified by the U.S. Food and Drug Administration (FDA). We are the only Chinese CPAP brand with FDA-certified products that are genuinely sold in the United States, ranking second in U.S. market share. We are also the first Chinese medical device company to successfully respond to a U.S. Section 337 investigation.
World's 1st international standard for COVID-19 & acute respiratory diseases.
PAP Link Digital Health solution, comprising Web, PC and APP versions, provides you with a complete set of tools to increase efficiencies by streamlining workflows, drive better patient outcomes through systematic management, and boost their therapy comfort.
· YiheJiaye(BMC) established.
· Core team formed.
· Launched the first-generation sleep diagnostic product and CPAP device.

· Obtained CE certification, enabling entry into global market.
· Completed Series A financing of RMB 8M.
· Launched the G1 BPAP series.
· Obtained FDA clearance and officially entered the U.S. market.
· Established the first Sleep & Respiratory Health Experience Center.

· Established Shenzhen R&D Center.
· Established BMC (Tianjin) Medical Co., Ltd.
· Completed Series B financing of RMB 60M.
· Launched the Respiratory Health Data Cloud Platform.
· Successfully addressed the U.S. Section 337 investigation.

· Established the first Customer Service Center.
· Completed Pre-IPO financing of RMB 37M, preparing for capital market entry.
· Launched the globally best-selling G3 BPAP series and 1st-generation medical ventilators.
· CPAPs production exceeded 1 million units.

· Listed on Shenzhen ChiNext (301367.SZ).
· Established Dongguan Production Base.
· Launched oxygen concentrators and medical cylinders, officially entering the oxygen therapy market.
· Established subsidiaries in France (BMC Medical France SARL) and Hong Kong.
· Launched E5/P5 CPAP series.

· Renamed to BMC Medical Co., Ltd.(formerly "YiheJiaye"), achieving unified corporate, securities, and brand naming.
· Launched E5/P5 CPAP for pulmonary diseases.
· Launched the KO1 portable oxygen concentrator, building a smart oxygen therapy branch.
